Sodium Hypochlorite Storage Tanks are manufactured from HDPE, XLPE, and FRP materials at 1.9 specific gravity. Secondary containment is required. Bleach is best stored out of direct sunlight, at temperatures below 60°F, filtered and diluted with pH kept above 10. Tank capacities range from 35 to 100,000 gallons. Prices range from $300 to $150,000.
- Commonly known as Bleach, Hypo, NaOCl, Sodium Oxychloride
- Used primarily for disinfecting and whitening
- There are 4 common storage tank materials used for sodium hypochlorite
- Federal guidelines outline correct handling and storage requirements
- Always get direct manufacturer approval before using any container

What is Sodium Hypochlorite?
Known commonly as bleach, sodium hypochlorite is an ionic compound with unique properties that is regularly used in many industries throughout the world. It is manufactured using chlorine gas and sodium hydroxide and is a pH-dependent alkaline solution. Concentrated bleach solutions have pH values greater than 11 and are considered corrosive and hazardous.
Sodium hypochlorite is the chemical compound known largely as bleach when dissolved in water. Pure anhydrous (without water) sodium hypochlorite is very unstable and subject to intense chemical, even explosive, reactions in the presence of heat, friction, or other chemicals and is not commonly utilized. A solution of sodium hypochlorite is a clear, yellow to green liquid with an immediately recognizable odor.
Molecularly, sodium hypochlorite is the combination of a sodium cation, Na+, and the anionic compound hypochlorite, ClO-, from which the name sodium hypochlorite is derived:
Na⁺ + ClO- = NaOCl
The formula for sodium hypochlorite has several reported orientations, NaClO, ClNaO, and NaOCl, with perhaps the most common being the simple combination of the two ions; i.e. NaClO. Chemically, the oxygen atom creates the bond between the Na+ and ClO- ions to form the sodium hypochlorite ionic structure. Chemical formulas can be written in a variety of formats but here with sodium hypochlorite the preference is relative to the orientation or order of the molecular bonds, that is, NaOCl.
The manufacturing of sodium hypochlorite is frequently performed by reacting chlorine gas (Cl2), or its liquid form, with the caustic sodium hydroxide (NaOH), chilled and diluted. This process of making NaOCl is known as the Hooker Process and yields three products: sodium hypochlorite, water, and a salt, which here is sodium chloride (NaCl).
Cl₂(g) + 2 NaOH(aq) = NaOCl(aq) + NaCl(aq) + H₂O(l)
Most commonly, solutions are 12% NaOCl with a specific gravity of 1.2, demonstrating that sodium hypochlorite solutions are not chemically 100% NaOCl, but rather NaOCl diluted in water, with salt, and some sodium hydroxide left from the manufacturing process. The sodium hydroxide present in NaOCl solutions is generally in the range of 0.1% to 0.5% and serves as a buffer to keep the solution pH high in the greater than 11 range. This is important because when pH drops, NaOCl solutions begin to decompose into breakdown products, losing their effective strength.
How Should Sodium Hypochlorite Be Stored?
Bleach should be stored at conditions and in materials that accommodate the chemical's corrosive capabilities and instability. Sodium hypochlorite solutions are prone to decomposition, which reduces overall concentration and general effectiveness. Certain conditions and/or materials are best for storing bleach as they slow NaOCl breakdown and are resistant to its oxidative and corrosive activities.
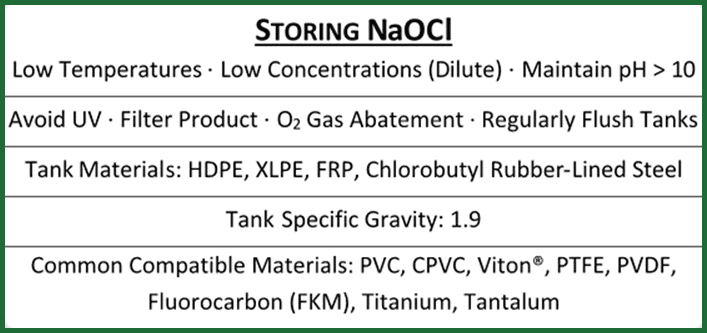 The following list outlines the most important recommended points related to sodium hypochlorite storage, tank materials, temperature, storage life, and requirements:
The following list outlines the most important recommended points related to sodium hypochlorite storage, tank materials, temperature, storage life, and requirements:
- Sodium hypochlorite is best stored for the longest storage life at temperatures around or below 60°F (15°C), when filtered and free of impurities, at dilute concentrations that maintain pH above 10, and without direct sun exposure due to the decomposition effects of ultraviolet radiation and heat.
- Recommended most common bleach storage tank materials are HDPE, XLPE, fiberglass reinforced plastic, and chlorobutyl rubber-lined steel. Sodium Hypochlorite tanks should be rated to 1.9 specific gravity. These tank materials are resistant to sodium hypochlorite corrosion.
- Popular recommended sodium hypochlorite storage tank component materials include PVC/CPVC pipes and fittings, Viton® gaskets, and titanium bolts. Other materials include: PTFE, PVDF, FKM and tantalum.
- Indoor bleach storage is preferred. If outdoor storage is required, tank systems should include components to address and mitigate UV exposure and solution heating to prevent sodium hypochlorite breakdown. Mastic coatings, insulation, and heat tracing are recommended options.
- Bleach products are most stable if filtered and stored at lower temperatures, diluted, and away from direct sunlight. Tanks should be flushed regularly to avoid accumulation of contaminates.
- All sodium hypochlorite storage systems should be vented for preventing buildup of oxygen (O2) off-gassing of NaOCl. Venting should be at least double the size of the inlet pipe diameter.
- Piping, fitting, and gasket materials should be resistant and semi-flexible; piping runs should be limited in extent as possible as an avoidance measure against oxygen-locking.
- For sodium hypochlorite storage regulations, secondary containment measures are a requirement for NaOCl storage conditions. Containment systems must be chemically resistant and capable of holding 110% of total system fluid capacity in the event of bulk material release.
- Bulk NaOCl storage containers should have their chemical resistance and specifications verified prior to applications.
Sodium Hypochlorite Storage Tanks
Sodium hypochlorite must be stored in specific containers and conditions in order to limit hypochlorite decomposition, ensure proper safety and tank stability, and to increase bleach’s useful service life. These factors are important and special considerations are warranted depending upon the solution’s concentration and other factors, such as purity. If not stored properly, a solution of NaOCl could potentially build up hazardous pressure from oxygen gas formation or could corrode through its container leading to structural failure. Secondary containment measures are required for NaOCl storage systems. Sodium hypochlorite is corrosive and dangerous and should always be handled and stored in accordance with all federally mandated regulations.
The concentration strength of sodium hypochlorite should always be considered when evaluating storage containers. Containers have manufacturer specifications only to certain levels that are dependent upon the chemical beyond which the container will lose its effectiveness. Snyder Industries, Inc. recommends NaOCl concentrations of less than 16.5% for high-density linear polyethylene (HDPE) tanks and recommends tanks with 1.9 specific gravity ratings and equipped with PVC fittings, Viton® gaskets, and titanium bolts. Concentrations greater than this are not safely compatible with this specific storage type as listed from this manufacturer, stressing the point that prior to applications, one should always receive manufacturer specifications concerning storage tank compatibility with the chemical to be stored and its concentration.
Four common types of storage containers are used for bulk sodium hypochlorite storage. The different types are listed in order of both their cost to the purchaser and the estimated service life of the container.
| NaOCl Storage Tank Comparison Chart | ||||
| Storage Container | Est. Service Life | Est. Purchase Cost | Est. Maintenance Cost | Considerations |
| HDPE / XLPE Tanks | 4 - 7 Years | $ | $ |
|
| Chlorobutyl Rubber-Lined Steel Tanks | 3 - 6 Years (Lining) | $$ | $ |
|
| Reinforced Fiberglass Plastic Tanks | 10 - 15 Years | $$ | $$ |
|
| Chlorobutyl Rubber-Lined Titanium | 30+ Years | $$$ | $ |
|
Polyethylene High-Density Linear and Cross-linked Tanks
High-density polyethylene (HDPE) or cross-linked polyethylene (XLPE) storage containers are the most cost effective variant but potentially have the shortest life expectancy. Cross-linked polyethylene is older technology than HDPE. XLPE tends to be more expensive, and a study by Exxon, Inc. showed that HDPE containers may store NaOCl more effectively than XLPE tanks. Both container types are fully capable of holding sodium hypochlorite solutions for anywhere from 4-7 years, if not longer. When seeking to get the fullest life expectancy out of any container, regular inspection and maintenance should be performed. Repair on HDPE / XLPE is limited however, where patching of holes or leaks, moving of an outlet, flushing and cleaning of the tank are types of maintenance / repair that can be performed. Maintenance can successfully postpone tank failure, extend service life, and effectively the time until a replacement unit is needed.
When storing sodium hypochlorite, polyethylene containers may best be suited for indoor, climate-controlled environments due to the decomposition tendencies of NaOCl. When storing NaOCl poly tanks outside, the tanks should be manufactured to inhibit heat-storing and the decomposition of NaOCl into O2 gas that occurs due to UV exposure. For outdoor storage, tanks are recommended to be made with a UV stabilized resin, such as Resin # 880059, and / or polyurethane foam insulation and mastic coatings equipped. Additionally, outdoor containers should be opaque and painted white to inhibit UV infiltration as well as dissipate the energy that can heat NaOCl solutions to decomposition levels. Vertical cylinder tanks with flat bottoms and domed tops are the most common type of poly containers used to store sodium hypochlorite. Many companies report very successful storage of sodium hypochlorite with polyethylene containers. Build your sodium hypochlorite tank and request a quote.
An additional consideration: due to the reported lifespans of NaOCl poly tanks, it is recommended that tank placement allows for easy tank replacement when the time for replacement occurs.
Chlorobutyl Rubber-Lined Steel Tanks
Chlorobutyl rubber-lined steel can successfully store solutions of sodium hypochlorite. These storage types are often chosen for large capacity tanks or ones that undergo regular fill and discharge cycles. Steel tanks are not as common as polyethylene or FRP tanks for smaller applications. These steel tanks are lined on the inside with a rubber lining made of 100% chlorobutyl rubber composition, not natural rubber. The lining must be installed by a skilled professional and cured with heat. These linings can last for 3 - 6 years but integrity inspections should occur regularly as any break in the rubber lining will expose the steel container to the corrosive activities of the sodium hypochlorite solution, which can lead to iron contamination and tank structural damage. The lining should be replaced promptly at the first sign of failing.
Chlorobutyl rubber linings are reported as providing superior resistance to sunlight, ozone, and weathering as well as resistance to chemical activities and permeation. This holds relevance when considering sodium hypochlorite’s decomposition sensitivity towards ultraviolet radiation and temperature. The chlorobutyl rubber linings are described as being heat resistant up to 200°F for most acids and caustics. These rubber linings can be applied to steel, titanium and fiberglass reinforced plastic storage tanks.
Fiberglass Reinforced Plastic (FRP) Tanks
FRP containers, or fiberglass reinforced plastic, (also reinforced fiberglass plastic, RFP), are more expensive than linear / cross-linked polyethylene tanks but can be more resistant to long-term corrosion and thus a longer service life. FRP tanks can make excellent choices for the storage of sodium hypochlorite. For the most successful and safe storage of NaOCl, an FRP container should be made with a proper internal corrosion barrier put in place, otherwise corrosion can be a serious issue. Vinyl resin is common for FRP structural layers as well as the corrosion barrier.
According to Powell Fabrication & Manufacturing, Inc., this storage type can provide up to 10 - 15 years of service life if properly constructed and maintained through regular tank inspections to ensure the integrity of the corrosion barrier. An improper design or lack of maintenance can lead to tank failure as soon as 3 - 5 years. Tank failures cause potential safety hazards and require costly complete replacements. The corrosion barrier should be made of a vinyl resin and be laid in an "ortho-wound" manner rather than a "filament-wound." Filament-wound styles are not recommended as it allows the sodium hypochlorite solution to slowly work its way around the continuous strands of glass, eventually leading to corrosion and failure of the container. Similar to polyethylene tanks, FRP is best suited for indoor, climate-controlled NaOCl storage. FRP tanks can be re-lined with replacement corrosion barriers, potentially extending tank service life.
Chlorobutyl Rubber-Lined Titanium Tanks
The final storage type on this list: chlorobutyl rubber-lined containers made of titanium. These storage containers are the most expensive and longest lasting, capable of a service life up to and beyond 30 years owing to titanium’s resistance to NaOCl’s corrosive activities. Commercial, pure grade 2 titanium is the grade commonly utilized for NaOCl applications. Due to the high cost of a titanium-made container, these storage containers are infrequently used and applications occur mostly in high-demand situations where equipment failures and repair down-times are not tolerable.
Bleach Storage Tank Components
Sodium hypochlorite storage tank components should be chosen based upon their NaOCl durability: incompatible materials can lead to hazardous and / or costly results. Sodium hypochlorite solutions are typically beneath 20% concentration strength and studies on compatibility reflect this. PVC fittings, titanium bolts, and Viton® gaskets are the top materials recommended for components in bulk NaOCl storage scenarios. EPDM gaskets are not highly recommended due to a reportedly relatively short service life when in contact with sodium hypochlorite solutions. Hastelloy-C® is reported as being resistant to the corrosive effects of sodium hypochlorite but can effectively contaminate the product with nickel, as it is a nickel-based steel alloy, which is problematic as even small amounts of nickel will drive the rapid decomposition of NaOCl. See the following chart that outlines some common materials’ NaOCl resistance:
| Sodium Hypochlorite Material Resistance Chart | |
| Material | NaOCl < Compatibility |
| 304 SS | Fair / Moderate |
| 316 SS | Fair / Moderate |
| ABS Plastic | Good / Minor |
| CPVC | Excellent |
| PVC | Excellent |
| HDPE | Excellent |
| LDPE | Excellent |
| XLPE | Excellent |
| Polycarbonate | Fair / Moderate |
| Polypropylene | Excellent |
| Nylon | Not Recommended / Severe |
| Neoprene | Fair / Moderate |
| PTFE | Excellent |
| PVDF (Kynar®) | Excellent |
| Tygon® | Fair / Moderate |
| VITON® | Excellent |
| Hastelloy-C® * | Excellent |
| EPDM | Good / Minor |
| Aluminum | Not Recommended / Severe |
| Brass | Fair / Moderate |
| Copper ** | N/A |
| Carbon Steel | Not Recommended / Severe |
| Natural Rubber | Fair / Moderate |
| Chlorobutyl Rubber | Excellent |
| Titanium *** | Excellent |
| * HASTELLOY-C IS A NICKEL-BASED ALLOY CAPABLE OF DECOMPOSING NAOCL SOLUTIONS THROUGH NICKEL CONTAMINATION
** COPPER IS NOT A RECOMMENDED MATERIAL DUE TO COPPER CONTAMINATION THAT WILL CAUSE NAOCL DECOMPOSITION. *** SOME SOURCES LIST TITANIUM AS SUSCEPTIBLE TO NAOCL; HOWEVER, THIS IS GENERALLY ONLY TRUE FOR HEATED SOLUTIONS AROUND 150 - 200°F |
|
Sodium Hypochlorite Storage Requirements and Chemical Compatibility
Successful sodium hypochlorite storage should include consideration of the chemical's characteristics, compatibilities, and the factors that contribute to NaOCl storage life and breakdown.
Sodium Hypochlorite Secondary Containment
Sodium hypochlorite storage regulation requires secondary containment measures in the event of catastrophic tank failure releasing bulk hazardous material. Good engineering practices direct containment areas should hold at least 110% the total volume of the tank or tanks being protected.
Poured concrete floors and walls are generally an effective means of secondary containment for bulk storage applications. Block concrete walls are not recommended due to the construction being more porous than its poured concrete counterpart. Paints or other types of sealants can be applied to concrete settings to further increase the material’s resistance to NaOCl leaching through the secondary containment. Note that no sealant or measure is 100% resistant to chemical leaching but some are more effective than others are.
HDPE catchment basins are secondary containment measures applicable for smaller NaOCl storage tanks. Double-walled tanks are another secondary containment option for sodium hypochlorite solutions and make good selections when operational spaces may be limited for full tank and external containment measures.
Specific laws and regulations may not explicitly require the installation and maintenance of NaOCl secondary containment but it is suggested as a requirement due to the hazards of the chemical and the volumes generally in question.
Sodium Hypochlorite Specific Gravity and Freezing Points
Sodium hypochlorite specific gravity ratings vary directly with the percent of excess sodium hydroxide remaining from the manufacturing process. The more sodium hydroxide, the greater the specific gravity ratings will be for NaOCl solutions. The following data for specific gravity was measured with a percent excess sodium hydroxide around 0.2%, a common percentage from today’s NaOCl production process. Note some approximations have been made.
| Sodium Hypochlorite Specific Gravity & Freezing Point | ||
| % Sodium Hypochlorite | Specific Gravity | Freezing Point |
| 4% | 1.06 SG | 24°F |
| 6% | 1.09 SG | 18.5°F |
| 8% | 1.12 SG | 17°F |
| 10% | 1.15 SG | 7°F |
| 12% | 1.18 SG | -3°F |
| 14% | 1.21 SG | -14°F |
| 16.5% | 1.25 SG | -17°F |
Compatible and Incompatible Metals
Sodium hypochlorite is only compatible with a short list of metals that includes titanium, platinum, gold, silver, and tantalum. All other metals will result in NaOCl solution contamination, which will cause an increase in product decomposition and oxygen gas production. Stainless steel, copper, brass, Hastelloy®, and Monel® metals should be avoided in NaOCl applications. Copper is perhaps the most notable in this list due to its increased usage in industrial piping. Copper piping can cause elemental copper (Cu2+) to contaminate NaOCl solutions and cause a marked increase in product degradation.
NaOCl Storage Life, Temperature, and Decomposition
Sodium hypochlorite will decompose over time, but certain conditions increase storage life by minimizing this breakdown rate. Concentration, temperature, purity, and UV exposure all affect NaOCl storage life. There are two bleach decomposition pathways: a primary pathway and an alternative pathway.
The primary pathway leads to the formation of sodium chlorate, NaClO3, which reduces the amount of hypochlorite that is available for use in its applications. The alternative pathway is catalyst-dependent. A catalyst drives the breakdown of NaOCl to sodium chloride salt and oxygen gas. The alternative pathway reduces the amount of molecular oxygen present and subsequently the amount of hypochlorite, diminishing the strength of the bleach.
Primary Pathway: 3 NaOCl = 2 NaCl + NaClO₃
The primary decomposition pathway occurs as a function of time that is dependent on the variables of concentration and temperature. NaOCl decomposition occurs as a second order rate against the solution’s concentration, meaning that for every doubling of strength, the rate of decomposition will increase by a factor of four: Rate = K[OCl-]2. This particular effect can be inhibited by diluting the solution to a less potent concentration. A common practice is to dilute NaOCl with pure water upon receipt and storage as less potent solutions are more stable. It should be noted that diluting with water should be done limitedly to avoid lowering solution pH too far and should be done with soft, filtered water to avoid introducing metals or other impurities such as calcium or magnesium that can contribute to NaOCl breakdown.
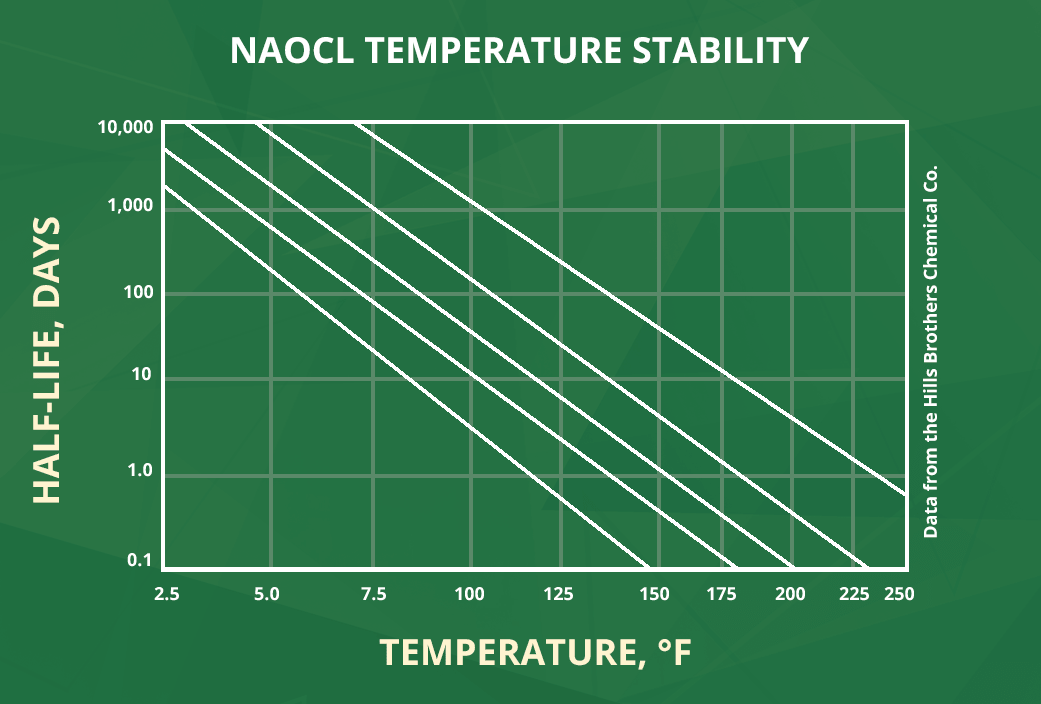
The second important variable contributing to NaOCl decomposition via the primary pathway is temperature. Estimations are that for every 10°C increase, decomposition rate will also increase by a factor of 3.0 - 3.5 per day. Therefore, it is recommended to retain solutions of NaOCl in climate controlled areas kept around 60°F (15°C) as the decomposition rate at this temperature, or lower, is greatly reduced. The NaOCl Temperature Stability chart, with data from the Hill Brothers Chemical Co., depicts how quickly a solution of NaOCl will degrade at different temperatures and solution strengths. The chart, from left to right on the x-axis, lists increasing temperatures, from bottom to top on the y-axis, increasing stability reported in molecular half-life, and plotted on the graph from left to right, the stability of different solutions in order of decreasing concentrations. In summary, the data demonstrates that a NaOCl solution is most stable at low temperatures and low concentrations.
The sodium chlorate formed during primary decomposition presents a potential problem along piping lines. Over time, excessive sodium chlorate can build up in the system and form crystals of sodium chlorate. To prevent this, regular flushing of all storage systems and piping is recommended.
Alternative Pathway: 2 NaOCl + Catalyst = 2 NaCl + O₂
The alternative pathway is catalyst-dependent, causing the direct decomposition of sodium hypochlorite. The catalysts of this reaction are sunlight’s ultraviolet radiation (UV) and certain transition metals, largely nickel, cobalt, copper, and iron. Direct sunlight emits electromagnetic waves across a broad solar spectrum with ultraviolet being the highest in energy and this electromagnetic energy is enough to stimulate the NaOCl molecule into releasing its oxygen atom, successfully catalyzing sodium hypochlorite to form NaCl and O₂ gas.
Sodium hypochlorite metal contamination can occur through residual impurities in the sodium hydroxide that is used in the manufacturing process, by using copper piping and stainless steel fittings, by using hard water during manufacture or dilution, and/or by not filtering the sodium hypochlorite product as this will leave residual contaminants in solution. A product of 50 PPB (parts per billion) or less of transition metals is considered to be a high purity product. Sodium hypochlorite solutions that have visible particles in solution or that are discolored from the traditional greenish-yellow are considered to be low purity products. The decomposition of NaOCl should be expected and accommodated for in such solutions.
The transition metals that contribute to the alternative pathway have greater reactivity with NaOCl according to the following order: Ni2+ > Co2+ > Cu2+ > Fe3+. Nickel catalyzes the reaction with the greatest effectiveness of the listed elements. Copper is generally a more common contaminant than cobalt and its effect on decomposition is much less than nickel’s. Having both nickel and copper present seems to increase the overall catalytic reaction rate even further than when the elements are present individually.
The presence of organic material or suspended solids of calcium or magnesium can also increase the rate of decomposition and bears the point that sodium hypochlorite solutions that have been filtered as part of the manufacturing process are best because particulate filtering will also remove some of the transition metals that contribute to decomposition by the alternative pathway.
In summary, sodium hypochlorite storage life is best for pure, lower concentration solutions kept at temperatures beneath 60°F (15°C). For every 10°C increase, decomposition rates can increase by a factor of 3.0 - 3.5 per day. Metal contamination and UV should be avoided for successful storage.
Oxygen Off-Gassing & Tank Venting
The O2 gas formed from NaOCl decomposition presents a problem not only in the piping and usage of sodium hypochlorite, but also in the storage of the chemical. If O2 gas production occurs under storage, pressure buildup can occur. This then presents the safety concern of handling a hazardous liquid kept under pressure. Sodium hypochlorite storage tanks should have a venting system to allow the release of built-up oxygen gas and should receive regular maintenance / inspections to ensure the release of any accumulated pressure. A general recommendation for venting is to use vents of diameters at least double the inlet pipe diameter. The use of flexible joints in pipework is recommended to accommodate for potential contraction and expansion that can accompany chemical off-gassing. For similar reasons, NaOCl piping runs should be limited in extent in order to limit the potential of "oxygen-lock," (see Pamphlet 96, The Chlorine Institute)
It is also recommended to use storage containers equipped with an outlet valve that allows for full draining of the sodium hypochlorite tank for maintenance and before refilling with fresh sodium hypochlorite. This flushing will prevent the buildup of nickel, cobalt, copper, iron and other suspended solids that contribute to the decomposition of NaOCl. Without regular flushing, these metals can accumulate in the container and can contribute to the very rapid decomposition of a sodium hypochlorite solution and subsequently the generation of oxygen gas.
What is Sodium Hypochlorite Used For?
The chemical solution of sodium hypochlorite is used extensively across the world for its ability to function as a whitening agent, an oxidizer, and as a sanitizing disinfectant. It sees heavy use in textiles, paper, detergents, petrochemical refining, and water / wastewater treatment industries. Plant matter such as cotton and wood fiber / pulp are processed with NaOCl to brighten or render the end product white.
Sodium hypochlorite is manufactured in the detergent industry for the cleaning and whitening of clothes, and is produced by brands such as Clorox®, Purex®, Hychlorite®, Anti-Formin®, and B-K Liquid®. Most companies do not produce the NaOCl they need on site but rather purchase it from a manufacturer. Companies such as Jones Chemicals, Inc. and Hawkins Chemical, Inc. boast having been in the business of producing NaOCl since 1930 and 1938, respectively. Companies such as Powell Fabrication & Manufacturing, Inc. sell the equipment necessary for the production of NaOCl.
The petrochemical industry uses sodium hypochlorite for the cleaning of certain critical equipment areas where strong acids cannot be used. Water and wastewater treatment plants use NaOCl to abate odors and to neutralize bacterial growth in water. Wastewater plants that handle toxic, cyanide-containing water, as from electroplating reactions, use sodium hypochlorite as an oxidizing agent to oxidize the harmful cyanide into the non-toxic cyanate.
A large industrial focus area for the usage of NaOCl is disinfection. Sodium hypochlorite is reportedly successful at the elimination of Clostridium difficile spores and the norovirus, where other common cleaning agents, such as quaternary ammonium compounds, are not. NaOCl is used to sanitize equipment prior to use, especially in the healthcare and food processing professions. For the most effective disinfection, a diluted NaOCl solution should be used and surfaces rinsed or allowed to drain.
Interesting Facts About Sodium Hypochlorite:
- A dilute solution is used in endodontics when a tooth root canal has become infected.
- NaOCl is used as a neutralizer of specific chemical warfare nerve agents after exposure.
- NaOCl is a better disinfectant if diluted, and the CDC, APIC, and OSHA recommend the use of NaOCl for disinfection.
- Neutrophils (immune system cells) within the human body produce trace amounts of sodium hypochlorite to destroy invading threats such as viruses and bacteria.
NaOCl PH, Safety and Handling
The solution of sodium hypochlorite is very pH dependent and exists in an equilibrium, shown below, that balances more to one side depending upon the pH of the solution.
Cl₂ + H₂O ↔ HOCl ↔ OCl-
← Decreasing pH | Increasing pH →
When the pH of such a solution begins to fall beneath a pH of 10, such as through dilution or adding an acid, the sodium hypochlorite molecule begins to decompose into hypochlorous acid, HOCl. When the pH of a sodium hypochlorite solution falls beneath a pH of 4.5, the solution’s equilibrium shifts to favor the generation of free Cl2, which poses health hazards as free, gaseous Cl2 is toxic. This potential for Cl2 production is why buffering sodium hypochlorite solutions is important. NaOCl solutions are buffered by a small amount of sodium hydroxide and/or sometimes by sodium carbonate to help retain the solution’s pH above 10 and increase the solution’s overall resistance to pH changes.
Hypochlorous acid is a decomposition product of NaOCl that occurs as pH decreases from being alkaline towards a neutral pH of 7. Concentrations of HOCl are greatest in the pH range of 4.5 to 6.5, beyond this the equilibrium begins to shift towards the other chemicals. Hypochlorous acid is a strong oxidizer and effective disinfectant. HOCl is why a dilute bleach solution is a better disinfectant: by diluting bleach with water, the pH is decreased to favor the generation of hypochlorous acid. Hypochlorous acid itself is unstable and readily decomposes into one of two sets of potential products: the first set is hydrochloric acid (HCl) and chloric acid (HClO3) while the second set is HCl and oxygen gas.

Overall, a sodium hypochlorite solution is an alkaline solution, (having a pH > 7), and presents increasing safety concerns with stronger concentrations of the chemical. Domestic use bleach purchased for in-home use traditionally ranges from 3 - 10% NaOCl with the remaining ingredients being mostly water, salt, and some sodium hydroxide or sodium carbonate.
Even diluted forms present safety concerns and can be severe eye and skin irritants that need to be handled properly and safely and should not be mixed with other chemicals unless done so by a professional fully aware of the very real hazards. When handling concentrated or industrial bulk quantities of sodium hypochlorite, full personal protective equipment should be worn as outlined by supplier-provided Safety Data Sheets relevant to the specified NaOCl solution strength. Always follow regulated safe-handling recommendations.
Specific warning do not mix bleach with ammonia-containing products. Common products that may contain ammonia are all-purpose kitchen and bathroom cleaners, glass cleaners, and oven cleaners. Mixing sodium hypochlorite with ammonia will drive the formation of chloramine vapor, which is very toxic. Similarly, and in additional warning, mixing bleach with an acid, (such as a toilet-bowl cleaner), will lower the pH to favor the production of toxic chlorine gases and should be avoided.
Sodium Hypochlorite Storage Takeaways
Always communicate your needs and specifications directly with the tank manufacturer whenever purchasing storage tanks for holding sodium hypochlorite. Acquire each individual manufacturer’s direct approval as maximum concentration ratings may differ, even for the same materials due to differences in construction, and as NaOCl strength can be measured and reported in various ways. Always verify with tank manufacturers that the container can safely meet the specific conditions and needs. Follow federal safety guidelines when handling NaOCl, (see the CDC for safety information concerning NaOCl solutions with Active Cl > 10% and with Active Cl < 10%).
Sodium hypochlorite is a useful, somewhat versatile, and widely used chemical. It is corrosive and dangerous, especially in greater concentrations when the pH exceeds that of eleven. The chemical as dissolved in water can decompose via two separate pathways and these reactions can occur quite rapidly given the right conditions of solution strength and temperature. When purchasing a storage container, consideration should be given to the factors that contribute to decomposition, O2 production, and to the chemical characteristics and hazards of sodium hypochlorite.
Protank is a national supplier of chemical storage tanks nationwide. Buy Sodium Hypochlorite tanks for sale at commercial and wholesale prices. Storage containers are designed and customized to meet your requirements. Contact us to see how we can help.
 by Alek Eccles
by Alek Eccles
Protank Chemical Extraordinaire
